|
|
  |
| WHAT
IS KERYDIN (tavaborole) ANTIFUNGAL TOPICAL NAIL SOLUTION? |
| Kerydin
Nail solution is a NEW, medical-breakthrough. first of a new class of antifungals
utilizing Boron technology. Kerydin is a FDA approved TOPICAL nail solution
for TOENAIL fungal infections and is active against most strains of Trichophyton
rubrum and Trichophyton
mentagrophyts.
Kerydin topical solution is applied via a dropper, around and under the
toenail - it reaches the site of onychomycosis to fight the fungus that
lives beneath the nail. The active ingredient in Kerydin (tavaborole) Topical
Solution cream is tavaborole 5%, specifically formulated to reach the site
of onychomycosis, to treat toenail fungus. Unlike Lamisil tablets
nail fungal medications taken orally which can affect the entire body,
Kerydin (tavaborole) Topical Solution works from the outside directly on
the nail fungus. Lamisil tablets can result in liver failure. Nail the
infection from the outside in with Kerydin Topical Nail solution prescription. |
| WHAT
IS A NAIL FUNGUS INFECTION, ONYCHOMYCOSIS? |
| Onyhomycosis
or nail fungus infection is a medical condition that is treatable.
Nail fungal infections are caused by dermatophytes, a fungus that infects
skin, nails and hair. Kerydin is FDA approved only for the treatment of
TOENAIL infections. Most often the fungus enters just under the top of
the nail and moves steadily toward the cuticle, the white half moon area.
The infected nails become discolored, yellowish-brown, thickened, misshaped
and eventually (if left untreated) separate from the nail bed and the nail
crumbles off. |
| WHO
IS SUSCEPTIBLE to NAIL FUNGAL INFECTIONS? |
| Over
30 million Americans are affected. Nail fungus infection is most
commonly seen on the toenails of men and the fingernails of women.
Most infections occur in those between the ages of 40 to 65 but anyone
can become infected. Some individuals have an inherited tendency to have
nail fungal problems. Also, individuals with a compromised immune
system and certain types of long-term conditions such as HIV-positive,
diabetes etc. are at an increased risk. The following are some environmental
factors increasing the risk of nail fungal infections: Continuous exposure
of hands and feet to moisture, shoes fitting too tightly, not changing
shoes often enough, injury to toe or fingernail. Kerydin is FDA approved
for the treatment of TOENAIL fungus. |
WHY
DO I NEED TO TREAT A MILD NAIL INFECTION?
IF LEFT UNTREATED
WILL IT GO AWAY BY ITSELF? |
| Onychomycosis
is a progressive fungal infection. The fungus destroys tissue and
organic debris accumulates under the nail leading to nail changes beginning
with slight thickening and discoloration. If left untreated the nail
plate eventually separates from its nail bed and crumbles off and the infections
spreads to other nails. The condition progresses from what initially
appears to just be a "cosmetic" discoloration of the nail to a painful
debilitating condition affecting ones ability to walk or use their fingers. |
|
WHO QUALIFIES
FOR AN ONLINE KERYDIN CONSULTATION?
|
| If
you have previously been diagnosed by a health care profession as suffering
from mild to moderate nail fungal infection (click
here for photos of nail fungal infection) you
may finally have find a safe and simple solution to the embarrassing condition
of discolored, thickened and hardened nails due to a dermatophyte fungal
infection. Kerydin antifungal nail solution is applied directly at the
site of the fungal infection and may work for you when other treatments
have failed. Kerydin prescription is not for those with severe fungal
infections involving the entire nail. Mild to moderate infections are those
involving only several nails and more than half of the nail may be affected
but NOT including the cuticle white half moon area at the top of the nail
where it joins onto the finger. If you have many nails affected, the entire
nail is involved, brittle and has lifted off from the nail bed and is accompanied
by ulceration of surrounding skin and pain you do NOT qualify for an online
consultation, you must seek immediate medical attention from your own physician.
If you have long-term conditions affecting your immune system such as diabetes,
organ-transplant recipient, HIV-positive, regular user of steroids or steroid
inhalers you do not qualify for an online consultation. |
|
WHEN &
HOW OFTEN DO I USE KERYDIN (tavaborole) TOPICAL SOLUTION?
|
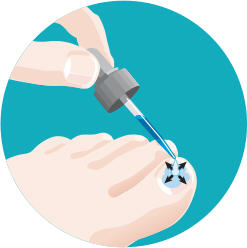
| Kerydin
Topical Solution is applied only once a day to the top and underside of
the toenail of the affected nails with the dropper provided for 48 weeks.
Once daily,
set
a schedule -
When you wake up in the morning or After you shower* or Just
before bed
*Wait
for at least 10 minutes after showering, bathing, or washing before applying
Kerydin. |
|
HOW LONG BEFORE
I SEE RESULTS?
|
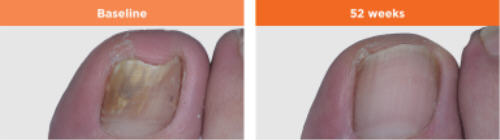 Nail
infections are very difficult to cure and it may take many months of treatment
to see the effects as toenails grows very slowly. Toenails can take 12
months or longer to completely regrow. Additionally, the time it takes
for a healthy nail to grow back varies from person to person. Even after
the fungus has been eliminated, nail regrowth can sometimes take a year
or longer. But don't despair and discontinue treatment if you
do not see improvement right away. It may take 6 months or longer to start
to see improvement. 48 weeks of treatment is required for a cure which
is defined as 10% or less residual nail involvement.
Nail
infections are very difficult to cure and it may take many months of treatment
to see the effects as toenails grows very slowly. Toenails can take 12
months or longer to completely regrow. Additionally, the time it takes
for a healthy nail to grow back varies from person to person. Even after
the fungus has been eliminated, nail regrowth can sometimes take a year
or longer. But don't despair and discontinue treatment if you
do not see improvement right away. It may take 6 months or longer to start
to see improvement. 48 weeks of treatment is required for a cure which
is defined as 10% or less residual nail involvement. |
|
HOW IS KERYDIN
DIFFER FROM THAN OTHER NAIL FUNGAL TREATMENTS?
|
| Nail
fungus are very difficult to clear up and if left untreated will only progress.
Kerydin (tavaborole) Topical Solution differs from oral medications because
it is applied directly to the site of the infected nail and it does NOT
have systemic effects. Since antifungal nail treatment needs to be
continued for many months, Kerydin is advantageous as it has no systemic
toxicity and is much better tolerated and safer than other available treatments.
Kerydin is a NEW FDA-approved topical prescription treatment proven effective
for treating mild to moderate TOENAIL fungal infections in immunocompetent
candidates. Kerydin is NOT approved for fingernail infections. |
|
HOW DOES KERYDIN
DIFFER FROM PENLAC NAIL LACQUER or KERYDIN SOLUTION?
|
| Ciclopirox
nail lacquer (Penlac) was the only topical treatment approved by the Food
and Drug Administration for the treatment of onychomycosis in the United
States, until Jublia solution was FDA approved. Penlac (ciclopirox) nail
lacquer has reported complete cure rates of only 5.5-8.5%, requires frequent
nail debridement and patients have to use alcohol to remove excess buildup
of lacquer from the ventral aspect of the nail plate to avoid additional
infection. Jublia has shown complete cure rates of 15-17%.
Kerydin (tavaborole) is now the NEWEST antifungal prescription approved
by the FDA and represents the next generation of topical therapies for
at-risk patients. Kerydin is the first of its kind utilizing Boron technology
and works differently based on its novel antifungal MOA and has demonstrated
up to 60% clinical improvement.
KERYDIN MECHANISM
OF ACTION: Inhibits Protein Synthesis
Tavaborole’s
structure contains a boron atom, which enables it to trap tRNA within the
editing site of Leucyl-tRNA synthetase (LeuRS).
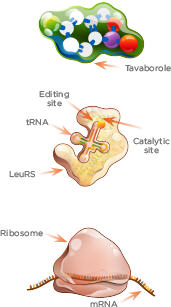
1.
LeuRS loads amino acid onto the tRNA at the catalytic site.
2.
LeuRS checks accuracy of aminoacyl-tRNA at the editing site.
3.
Tavaborole targets fungal cytoplasmic LeuRS by binding to the editing site
together with tRNA. There, tRNA is trapped.
4.
Because tRNA cannot complete the amino acid transfer to the ribosome for
assembly, protein synthesis is effectively blocked.
|
|
CAN
KERYDIN TOPICAL SOLUTION BE USED WITH OTHER ANTIFUNGAL NAIL TREATMENTS
OR BE USED WITH NAIL POLISH?
|
| Kerydin
Topical solution is NOT recommended to be used with any other systemic
antifungal agents for nail fungus infection as there are no studies to
determine their interactions. Also DO NOT use nail polish, acrylic
nails, or any other cosmetic nail products during treatment regimen with
Kerydin. Avoid pedicures, use of nail polish and cosmetic nail products
while using Kerydin. |
| WHAT
IF I MISS A KERYDIN (tavaborole) TOPICAL SOLUTION APPLICATION? |
| If
you miss a Kerydin (tavaborole) Topical Solution application it is best
to just skip the missed dose and continue on with your daily nail care
regimen. Never double-up or use a greater amount to make- up
for missed dose, this may only enhance the likelihood of more severe skin
irritations. |
| WHAT
ARE THE SIDE EFFECTS OF KERYDIN (tavaborole) TOPICAL SOLUTION? |
| Kerydin
Nail solution does not have systemic effects when used appropriately.
There are No know drug interactions and No laboratory monitoring is needed.
The majority of patients using Kerydin (tavaborole) Topical Solution experience
no side effects, and any side effects are mild if any. The most common
side effects seen during clinical trials were ingrown toenails, redness,
itching, swelling, burning or stinging, blisters, and pain. Kerydin
may cause other side effects. If nails start to change shape or you
have prolonged swelling and pain around your nails, discontinue use and
contact your regular doctor. If you get the medication in your eyes,
rinse immediately with water and seek immediate emergency treatment!
If you have any signs of an allergic reaction to Kerydin: hives; difficult
breathing; swelling of your face, lips tongue, or throat seek immediate
emergency medical help. |
WHO SHOULD NOT USE KERYDIN
NAIL SOLUTION?
WHAT ARE THE CONTRAINDICATIONS
FOR KERYDIN (tavaborole) Topical Solution?
|
-
Do NOT use
Kerydin (tavaborole) Topical Solution if you are pregnant or attempting
to become pregnant or breast feeding.
-
Do NOT use
Kerydin (tavaborole) Topical Solution if you are breast feeding.
-
Do NOT use
Kerydin (tavaborole) Topical Solution if you are allergic to any of the
ingredients: tavaborole in a solution base alcohol, propylene glycol, edetate
calcium disodium
-
Do Not use
on skin other than that immediately adjacent to infected TOENAIL
-
Do NOT use
on advanced severe fungal nail infections involving the entire nail
-
Do NOT us if
have a history of immunosuppression, history of recurrent shingles, on
cancer chemotherapy, HIV-positive, organ transplant recipient, diabetic,
diabetic neuropathy - you need to be constantly monitored by a physician
during treatment as there is high potential of complications and superinfection.
-
Do NOT use
in eyes. Do NOT use orally. Do NOT use on mucous membranes on intravaginal.
Do NOT use KERYDIN in your eyes, mouth or vagina.
-
Not approved
for pediatric use
-
Do NOT use
Kerydin on fingernails.
-
Kerydin FDA-approved
for the treatment of TOENAIL FUNGAL infections ONLY!
|
| We
will not prescribe Kerydin (tavaborole) Topical Solution to anyone under
the age of 18. Also Kerydin (tavaborole) Topical Solution should NOT be
used by pregnant women or women who are breast feeding. Kerydin (tavaborole)
Topical Solution is ONLY approved for use for fungal infections of the
toenails. |

|
|
|




